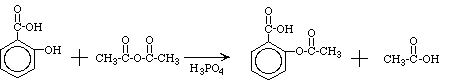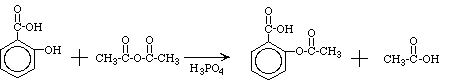Synthesis
of Aspirin
Aspirin, acetylsalicylic acid, can be easily made from salicylic acid by an
organic reaction know as esterification. Specifically in this lab, aspirin
will be prepared by allowing salicylic acid to react with acetic anhydride in
the presence of phosphoric acid, which is a catalyst for the reaction. The reaction
equation is shown below:
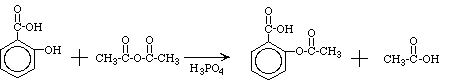
Salicylic acid Acetic anhydride Aspirin
Acetic acid
- Weigh a clean, dry test tube in a flask to 0.001
g.
- Add your charge of salicylic acid into the test
tube and reweigh in the flask (the mass difference, which is the same as the
mass of salicylic acid should be around 0.42 g).
- To the test tube, add three drop of 85% phosphoric
acid and 0.9 milliliter acetic anhydride from a calibrated plastic dropper.
- Shake the test tube to mix the reactants thoroughly.
Clamp the test tube in a water bath maintained between 90° and 100°C, and
heat. The contents in the test tube should dissolve; continue heating for
five minutes after the contents of the test tube are in solution.
- With another calibrated plastic dropper, cautiously add 0.5 mL of water
dropwise to the mixture to destroy the excess acetic anhydride. Then add another
1 mL more water and allow the test tube to cool to room temperature.
- You may see the formation of white crystals. If
crystals do not appear, scratch the inside of the test tube to get crystallization
started. Cool the test tube in an ice bath for at least ten minutes. Also,
chill about 5 mL of distilled water in another test tube in the ice bath.
- Weigh a piece of filter paper to 0.001 g. Collect
the crystals by suction filtration on a Buchner funnel (refer to the equipment),
wash the crystals with two 1 mL portions of thoroughly chilled water, and
suck the crystals as dry as possible by drawing air thorough them.
- Take out the crystals and the filter paper carefully
and put them on a watch glass. Leave them in your drawer to dry over the week.
Weigh the crystals and the filter paper during the next lab period. Determine
your percentage yield (Hint: Salicylic acid is the limiting reagent in the
reaction).