Percent Strontium Chloride Hexahydrate in a Mixture
Identification of elements or compounds is called qualitative
analysis. Determination of the AMOUNT of a substance present in a sample is
called quantitative analysis. Methods of quantitative analysis include gravimetric
(weighing), volumetric (titration) and instrumental. Whatever the method the
final calculation is the same:
mass % A = (mass of A determined/sample
mass)Χ 100%
In a gravimetric method determination of component "A"
is based on the mass of some product formed on treating a weighed sample containing
component "A" and other ingredients.
If a
PURE sample of hydrate (salt nH2O) is heated
above its decomposition temperature the mass loss (difference in the masses
before and after heating) gives the mass of water originally hydrated in
the salt. If the identity of the anhydrous salt is known it is possible to
evaluate "n" by finding the moles of anhydrous salt, the moles of
water and calculating the ratio of moles of water to moles of anhydrous salt:
n = moles of H2O/moles of anhydrous
salt
In this specific experiment we already know the
value of n (n=6 for SrCl2 6H2O). However, the strontium
chloride hexahydrate (n=6) is mixed with inert substances in the sample. Inert
substances are substances that do not decompose or react in any way when the
sample is heated according to the directions given. By experimentally
find the mass loss upon complete dissociation, we can figure out the percentage
of the hydrate, SrCl2 6H2O, in the mixture sample
by using the same stoichiometric relationship discussed above.
The mass loss gives the grams of water lost by
the strontium chloride hexahydrate when it decomposes. The chemical equation
for the decomposition is:
SrCl2 6H2O --> SrCl2 + 6H2O
Thus
the amount of SrCl2
6H2O in the sample can be calculated from the number of grams
of water formed by converting grams of water to moles of water, converting moles
of water to moles of SrCl2 6H2O, and finally converting moles of SrCl2 6H2O to grams of SrCl2 6H2O:
Mass H2O = Mass loss on thorough
heating
Moles H2O = Mass H2O/
Molar Mass H2O
Moles SrCl2 6H2O = Moles H2O Χ (1 Mole SrCl2 6H2O/ 6 Moles H2O)
Mass SrCl2 6H2O = Moles SrCl2 6H2O Χ Molar Mass SrCl2 6H2O
% SrCl2 6H2O = (Mass.SrCl2 6H2O/sample Mass) Χ l00%
BEFORE COMING TO LAB USE THE FOLLOWING SAMPLE SET
OF EXPERIMENTAL DATA TO CALCULATE THE PERCENT STRONTIUM CHLORIDE HEXAHYDRATE
IN THE SAMPLE. Check your answer with the instructor on the day you do your
lab.
SAMPLE DATA
| mass of crucible, cover and sample |
26.804 g |
| mass of crucible, and cover |
24.304 g |
| mass of crucible, cover and sample (after 1st heating): |
26.002 g |
| mass of crucible, cover and sample (after 2nd heating): |
25.999 g |
|
CALCULATIONS:
|
_________% SrCl2
6H2O
|
- Do ONE trial CAREFULLY. Calculate
your percent SrCl2 6H2O and
check your answer with the instructor. If your answer is too far from the
correct value you will be asked to do another trial.
- You must use a clean crucible in this experiment
(if the "junk" on the crucible volatilizes during the heating process
your results will be worthless). CHECK THE CRUCIBLE CAREFULLY FOR CRACKS
BEFORE YOU START THE EXPERIMENT. If it is cracked get a new one from the
stockroom.

- Handle
the crucible only with tongs from this point on! (Fingerprints
will change the mass of the crucible and when a crucible is hot you don't
want to touch it with your hands). Wrap the tongs around the outside of the
crucible. Don't put one of the tongs inside the crucible (you will increase
the chance of contamination AND the chance of dropping the crucible). You
may carry crucible and cover in a clean porcelain evaporating dish.
- The crucible must be thoroughly dried BEFORE
putting the sample in it. To dry it set up an iron ring, place a clay triangle
over the iron ring, then support the crucible in the clay triangle. Place
the lid on the crucible leaving a small crack for the water vapor to escape
(if you put the lid on tight the escaping water vapor will probably pop the
lid off causing it to break). The clay tubes on the triangle may be bent
or broken to make the crucible fit in the triangle.
- Heat the crucible and its cover strongly
(with the bottom of the crucible near glowing red) for at least five minutes.
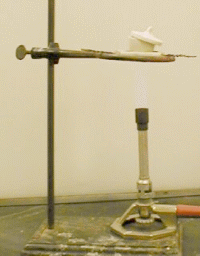
- Remove the burner and let the crucible
cool. After a few minutes it may be transferred to an asbestos pad if desired
but DO NOT SET A HOT CRUCIBLE ON THE DESK TOP (yes it will cool faster but
it will probably crack and you will have to start over. Also, considering
the small size, crucibles are very expensive).
- WHEN THE CRUCIBLE IS COOLED TO ROOM TEMPERATURE
weigh it on the Mettler Balance to the nearest milligram (0.00l g). Remove
the crucible from the balance.
- Add your unknown sample to the crucible.
Remembering to use tongs, place the crucible back on the balance and weigh
it again. Record all of your weighings DIRECTLY on the report form data sheet.
- Place the crucible (using tongs) back on
the clay triangle. Begin with gentle heating then heat more strongly. The
bottom of the crucible should be allowed to near glowing red for ten minutes.
Remove the burner and allow the crucible to cool as before.
- When it has cooled to room temperature
use tongs to handle it while you take it to the balance room. Weigh and record
the mass.
- Heat the crucible a second time for about
five minutes, cool and weigh. If the two masses agree to plus or minus five
to ten milligrams the decomposition is complete and you may calculate your
results. If the two weighings differ by more than plus or minus five to ten
milligrams you must heat strongly for five more minutes, cool and reweigh.
- Calculate your percent strontium chloride
hexahydrate and check your answer with the instructor. Be sure you use the
correct formulas in your calculation. Use atomic masses accurate to two decimal
places from the periodic table. Round your final percent to the number of
significant figures justified by the experimental data. Remember that treatment
of significant figures is different in addition and subtraction operations
than in multiplication and division operations. If your result is not close
to the true value list possible errors in your experimental procedure to explain
your poor result.



