In this experiment you will construct Lewis diagrams of several covalent compounds. You will then use the Lewis diagrams to make predictions regarding the structures of the molecules and build models based upon your predictions.You will predict the orbital hybridization of the central atom and decide whether the molecule is polar or nonpolar.
(This introduction section is very content intensive, which is divided into five smaller sections: Lewis Diagrams, Resonance, VSEPR Theory, Hybridization and Polarity. Even so, it is still just an overview of what you learned in two Chapters from your textbook. Therefore, you will find it essential to use corresponding chapters in your textbook.)
In order to construct a Lewis diagram you need to know how the atoms in a compound are connected to each other. Some general guidelines are the following:
1. The least electronegative atom, excluding hydrogen, is usually the central atom.
2. Carbon has four bonds, nitrogen three, oxygen two, and hydrogen and fluorine one.
3. The number of bonds for halogens other than fluorine varies: one if the halogen is the more electronegative atom participating in a bond, up to seven if it is the central atom.
4. Sulfur has two bonds if it is the more electronegative atom, up to six if it is the less electronegative central atom.
5. Often the more symmetrical of two diagrams is correct.
Begin by postulating a tentative order of attachment of atoms in the compound. Draw a line to represent each bond between atoms. Each line corresponds to an electron pair bond and therefore accounts for two electrons. Add unshared electron pairs to each atom except hydrogen to bring the number of electrons around it to eight (four pairs). Count the total number of electrons you have used in this process. Also count the number of valence electrons that are available. In general, each atom has a number of valence electrons equal to its group number. Thus carbon has four, nitrogen has five, and the halogens have seven. Compare the two numbers. If the number of electrons you have used exceeds the number available, you must decrease the number used by forming double and/or triple bonds between atoms. Since electrons used in a bond are counted for each atom involved in the bond, this will decrease the number of electrons that you need to give eight electrons around each atom.
There are certain cases in which less or more than eight electrons are placed around each atom. Only four electrons are placed around beryllium in its covalent compounds. Only six are placed around boron and aluminum. On the other hand, nonmetals in the third period and beyond may have up to twice their group number of electrons around them. Thus phosphorus may have as many as ten (five pairs) and sulfur as many as twelve (six pairs).
Electrons are paired if at all possible. If the number of available electrons is odd, a single electron must be used where normally an unshared electron pair would be required.
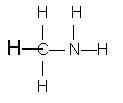
Example 1: CH5N
The least electronegative atom, excluding hydrogen, is carbon. Take carbon as the central atom. Since each hydrogen can have only one bond, you must attach the nitrogen to the carbon and the hydrogens to carbon or nitrogen to provide the correct number of bonds (four for carbon, three for nitrogen).
Postulated order of attachment of atoms:
H H The number of bonds used follows the guideline outlined
| | above: Carbon has four, nitrogen has three, and each
H—C—N—H hydrogen has one.
|
H
In the formulation shown, carbon already has eight electrons around it. The nitrogen needs an unshared pair of electrons to make up its complement of eight.

This structure uses a total of 14 electrons. The number available is 5 from hydrogen plus 4 from carbon plus 5 from nitrogen for a total of 14. This is an acceptable Lewis diagram.
Example 2: HCN
The least electronegative atom is carbon. Take it as the central atom. The postulated order of attachment is
H—C—N
Placing eight electrons around each atom except hydrogen gives
·· ··
H—C—N:
·· ··
The number of electrons used is 14. However, the number available is 1 from hydrogen plus 4 from carbon plus 5 from nitrogen for a total of 10. We must make a triple bond in order to use four fewer electrons. The structure is
![]()
Note that another approach would have been to use the valence guidelines to assign the number of bonds to each atom and then add unshared pairs as needed to provide eight electrons around each atom.
The least electronegative atom is boron. The postulated order of attachment of atoms is
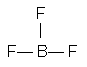
Placing eight electrons around each atom gives
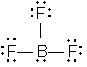
The number of electrons used is 26. The number available is 3 from boron and 7 from each fluorine for a total of 24. We might consider making a double bond between boron and one of the fluorines. We should not do that in this instance, however, because boron is one of the exceptions described above. Only six electrons are placed around it. Hence the correct Lewis structure is
Example 4: H2SO4
Sulfur is the least electronegative atom other than hydrogen. The postulated order of attachment of atoms is
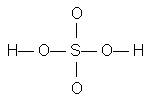
Two possible Lewis structures are
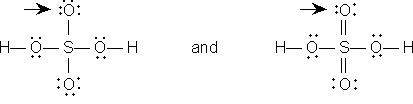
Both structures use 32 electrons. The number available is 2 from hydrogen, 6 from each of four oxygens and 6 from sulfur for a total of of 32. The structure on the right is allowable because sulfur can have up to twelve electrons around it. Note that this structure provides oxygen with its normal two bonds, whereas the structure on the left does not.
To decide which of the suggested structures is more
likely to be correct, we utilize the knowledge that the true structure of a
substance is more likely to be that obtained by minimizing formal charge.
The formal charge of an atom equals the number of its valence electrons less
the sum of the number of electrons in unshared pairs plus half the number in
bonded pairs. Thus for the oxygen atom highlighted by the arrow in the left
hand structure, the formal charge on the oxygen = ![]() .
For the same oxygen in the right hand structure, the formal charge =
.
For the same oxygen in the right hand structure, the formal charge =![]() .
.
A summary of the calculated formal charges on the left hand structure is given below. In the right hand structure the formal charge on all the atoms is zero.

Since the right-hand structure has no formal charges, it is the preferred Lewis structure.
Formal charge can also be used to decide between two possible ways of connecting atoms.
Two possible structures for HNO2 are
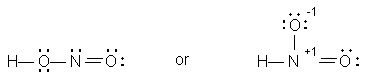
Since the left-hand structure has no formal charge, it is the more likely. Note also that both oxygens in this formulation have two bonds, whereas one of the oxygens has only one bond in the right-hand structure.
Occasionally there are instances in which more than one Lewis structure can be written for the same order of attachment of atoms. An example is nitric acid, HNO3. Two Lewis diagrams can be written, as follows:
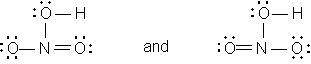
The distance between two atoms doubly bonded to each other is shorter than the distance between the same two atoms singly bonded to each other. It has been found experimentally, however, that the two nitrogen-oxygen bond distances in this compound (disregarding the bond to the oxygen that also carries a hydrogen) are identical and intermediate between normal single and double bond distances. Hence the true structure of nitric acid is a hybrid of the two Lewis structures. This is expected to be the case whenever more than one Lewis structure can be written for a species or substance.
Note also that N has four bonds in each of the structures. This is possible because it has a formal charge of +1. The singly bonded oxygen has a formal charge of -1.
Predicting Molecular Shape from Lewis Diagrams - VSEPR Theory
We can predict the shapes of molecules from Lewis diagrams using the Valence Shell Electron Pair Repulsion theory. This theory states that groups of electrons in molecules, being of like charge, repel each other, and therefore dispose themselves as far apart as possible. To make use of this theory, we need to know first, what is meant by a "group of electrons," and second, how various numbers of groups arrange themselves to maximize the distance between them.
Each of the following is considered a "group": A single bond, a double bond, a triple bond, an unshared pair of electrons, or a single electron.
To deduce the shape of a molecule, we determine how many groups of electrons are around its central atom. If the number is two, they are disposed at a 180° angle to each other. If it is three, the angle is 120°. Four groups are found at the corners of a tetrahedron, such that the angle between any two groups is 109.5°. Five groups are at the corners of a trigonal bipyramid at an angle of either 120° or 90°, depending on location. Six groups are at the corners of an octahedron, and the angle is 90°.
When there are one to three unshared pairs among five groups of electrons, repulsions are minimized by placing the unshared pairs in the triangular plane (Ex., Case II below).
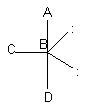
Observe the following overall shapes and the electron groups (represented by lines) that give rise to them. Note that two compounds that each have three atoms attached to the central atom have different overall shapes and geometries because the total number of groups of electrons is different.
Case I
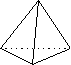

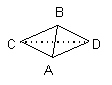
tetrahedron electron groups three atoms, one pair of electrons
on central atom; ABC angle ~109°
In Case I, the electronic geometry is tetrahedral; the molecule is shaped like a pyramid with a triangular base (as shown in the far righthand drawing)
Case II
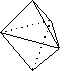
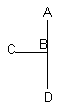
trigonal bipyramid electron groups three atoms, two pairs of electrons
on central atom; ABC angle ~90°
In Case II, the electronic geometry is trigonal bipyramidal. The molecule is T-shaped.
VSEPR theory gives us information about the location of atoms in a compound, but it does not explain how bonds are formed. Quantum mechanical theories have been developed to account for bonding in molecules. One of these, the valence bond theory, is based on the idea that bonds are formed by overlap of atomic orbitals. When atomic orbitals from each of two atoms overlap, the electron density of the electrons occupying those orbitals is concentrated between the two atoms and pulls their nuclei together, thus forming a bond.
In many cases, however, the ground state electron configurations of atoms derived from the Aufbau Principle do not permit overlap in directions that would form the molecular shapes that are actually observed. To account for the observed shapes, the concept of orbital hybridization is used.
Hybridization is the mixing of atomic orbitals in an atom to form a new set of atomic orbitals. Since orbitals are wave functions, this can be compared to mixing water waves to form new waves having shapes different from the originals. The hybridized orbitals of interest here are the following:
Orbitals Mixed Orbitals Obtained Angle between Orbitals
one s with one p two sp 180°
one s with two p three sp2 120° (pointing toward the corners of a triangle)
one s with three p four sp3 109.5° (pointing toward the corners of a tetrahedron)
one s, three p, one d five sp3d three in a plane at 120°,
two above and below the plane at 90°
(pointing toward the corners of a trigonal bipyramid)
one s, three p, two d six sp3d2 90° (pointing toward the corners of an octahedron)
Notice that the number of new orbitals is the the same as the number of orbitals mixed, and that the name of the new orbitals tells what orbitals were mixed to obtain them. Also notice that each kind of hybrid orbital is associated with one of the geometries predicted by VSEPR theory. Thus sp orbitals,of which there are two, are associated with the geometry expected for two groups of electrons; sp3 orbitals, of which there are four, are associated with the geometry expected for four groups of electrons. Therefore we can use VSEPR theory to predict the probable hybridization of the central atom in a compound. In Case I in the previous section, we would expect the hybridization of atom B to be sp3; in Case II above we would expect the hybridization of atom B to be sp3d.
Molecules that have separate centers of positive and negative charge are called polar molecules. It is easy to decide whether a diatomic molecule is polar. If the two atoms in the molecule are the same, they have the same electronegativity and share equally the electrons that bond them together. There is no separation of charge within the molecule and it is nonpolar. If the two atoms are different, their electronegativities are different. The electron density is greater in the vicinity of the more electronegative atom and it will be the center of negative charge, while the less electronegative atom will be the center of positive charge. Since there is a separation of charge within the molecule, the molecule is polar. A quantity called the dipole moment, µ, is a measure of the charge separation. It is the product of the magnitude of the charge, Q, and the distance between the charges, r.
µ = Q x r
For molecules containing three or more atoms, both the shape of the molecule and the polarity of the bonds must be considered when deciding whether a molecule is polar.
The dipole moment of the molecule as a whole is equal to the vector sum of the individual bond moments. The following examples illustrate this point. The unshared pairs on fluorine are omitted.
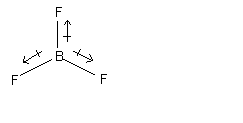
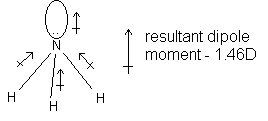
molecule is nonpolar molecule is polar
A. Pre-lab Assignment
Before coming to class, complete the following. Obtain your instructor's approval of your work before beginning the laboratory assignment.
1. Write a correct Lewis diagram for BF3 and derive the angle between any two F atoms and the B atom from the diagram. Also explain why the arrows that indicate the bond moments point in the directions shown.
2. Write a correct Lewis diagram for NH3 and derive the angle between any two H atoms and the N atom. Also explain the direction of the arrows indicating the bond moments.
3. Use the Lewis diagrams given in the introduction to derive:
the H-C-N angle and the C-N-H angle in CH5N
the H-C-N angle in HCN
the angle between S and any two O's in H2SO4. Is your prediction of the bond angle the same or different for the two possible Lewis structures?
the O-N-O angle and the N-O-H angle in HNO2
4. Are any of the compounds listed in part 3 nonpolar?
B. Model Building - Part I. Work in partners.
In this section, species are listed according to how many groups of electrons are found around the central atom. Assume that all formulas represent isolated molecules or ions. For each formula, draw a Lewis structure. Draw resonance forms if the formula represents a resonance hybrid. Use the Lewis structure to derive the electronic geometry, the hybridization of the central atom and the expected geometry of the species.
Make a model of each species using the models provided. Use long green bonds except when connecting hydrogen to other atoms, or making connections between atoms that are multiply bonded to each other. Use flexible white bonds for the second and third bonds in multiple bonds. Use the atom centers as directed in the attached table.
Make a sketch of the species. If it is a compound, show bond moments and decide whether the compound is polar . If it is polar show the direction of the compound's dipole moment.
Here is a list of which pieces to use for which atoms:
Atom Color Description of Piece
Atoms other than O, N, or S black tetrahedral
having tetrahedral geometry
C black tetrahedral for singly bonded C
trigonal bipyramidal for doubly bonded C
octahedral for triply bonded C
divalent metal black two prongs 180° apart
H white
singly bonded halogens green
N, elements having blue tetrahedral for singly bonded N or tetrahedral P
trigonal bipyramidal trigonal bipyramidal for doubly bonded N or
electronic geometry trigonal bipyramidal elements
O red one prong where O is bonded to one other atom
and that atom is in the third period or beyond
tetrahedral for singly bonded O
octahedral for O doubly bonded to second period
elements
S, elements having octahedral yellow two prong for S with trigonal electronic geometry
electronic geometry octahedral for S doubly bonded to second period
elements, or octahedral elements
tetrahedral for S with tetrahedral electronic
geometry
trivalent metal black three prongs 120° apart