Identification of an Unknown Liquid
Introduction
A
substance can be identified by observing its chemical and physical properties.
Physical properties are those that a substance can exhibit without undergoing a
change in chemical composition. Chemical properties are those that a substance
exhibits only by undergoing a change in chemical composition (i.e., a chemical
reaction).
The
identification of a substance by its physical properties is the more desirable
method because the sample is not destroyed in the determination. Some of the
more common physical properties are: color, odor, density, solubility, state
(solid, liquid, or gas at 20°C), melting point, boiling point, and refractive
index. Probably the major difficulty is that in order to determine accurate
values, one must be using a pure substance. Most materials found in nature are
not pure.
In this experiment you will
identify an unknown liquid (a pure substance) after measuring values of the
following physical properties:
- density
The density of a substance is a measure of its mass per
unit volume. Density units for liquids may be expressed in various sources as
g/mL, g/cm3, or g/cc, but all have the same numerical value. The
density of a liquid will vary with the temperature but this change is usually
negligible if the temperature change is small.
- boiling point
The boiling point of a liquid is the temperature at which its vapor pressure equals the external pressure, usually that of the atmosphere.
- refractive index
The index of refraction, expressed as nD20, is the ratio of the constant velocity of light in a
vacuum to the variable velocity of light in a medium. The number 20 represents
the Celsius temperature of the sample while D represents the monochromatic D
line of the sodium spectrum. The refractive index of a substance changes if the
temperature changes, or if the color of the light used changes. Refraction is
responsible for the bent spoon effect observed when a spoon is partially
submerged in water. Refractive index measurements can be used to determine
solution concentrations, ascertain purity and identify a compound.
Safety
Most
organic liquids are toxic, so you should exercise care whenever handling them.
Do not breathe the vapors. Do not allow unknown liquids to come in contact with
your skin. If this happens flush the area with copious amounts of water.
The unknowns are flammable.
Keep them away from all flames.
Procedure
1. Density determination of
an unknown liquid.
- Weigh a clean, dry, stoppered
50 mL Erlenmeyer flask as accurately as possible.
- Pipet 10.00 mL of the
unknown liquid into the Erlenmeyer flask. Replace the stopper avoiding any
contact between the stopper and the liquid. Weigh the Erlenmeyer flask,
stopper and added liquid on the same balance used for the initial
measurement.
- Calculate the mass of
the 10.00 mL aliquot of unknown liquid added to the flask.
- Calculate the density of the unknown to
four significant figures. This is necessary since many of the unknown
liquids in Table #1 have very similar densities.
- Once the density has
been calculated, try to eliminate compounds that have densities far from
this experimental value (of course, you need to be sure that your density
determination is accurate).
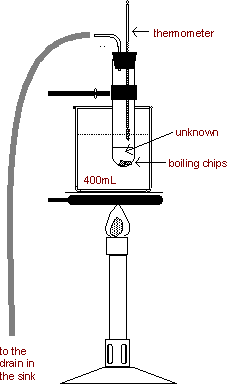 2. Boiling
point determination for an unknown liquid
2. Boiling
point determination for an unknown liquid
- Add approximately 5
mL of unknown liquid to a 25 X 200 mm pyrex test tube. A boiling chip may
be added to the liquid in the test tube to avoid bumping or uneven boiling
of the liquid.
- Close the test tube
with a rubber stopper fitted with a thermometer and a right -angle glass
tube.
- Run the rubber hose
from the bent glass tube into the sink to keep flammable vapors away from
the bunsen burner. The liquid
level should be so that the thermometer bulb is about 1 cm above the
surface of the liquid. Do not
attempt to slide the thermometer in the stopper; it will break!
- Clamp the test tube
to a ring stand. Support a 400 mL beaker half-full of water on an iron
ring and a wire gauze. The test tube should be immersed in the beaker of
water so that the unknown liquid is below the water level but the
thermometer bulb is above the water level. Use care to assure that the
thermometer does not touch the inside of the test tube and the test tube
does not come in contact with the beaker. See Figure above right.
- Heat the beaker of
water gradually. Record the temperature at which the liquid in the test
tube boils freely, then remove the burner.
- If you wish to
recheck this boiling point temperature, allow the water-bath temperature
to fall until the unknown liquid stops boiling, then reheat the beaker of
water. Again, using this experimental boiling point value, eliminate
unknown possibilities that have boiling points far from this determined
value.
3. Refractive index
determination for an unknown liquid.
- Before making a
refractive index measurement of the unknown liquid, your instructor should
brief you on the proper operating procedure for the refractometer. Next,
for practice, determine the refractive index of distilled water. When you
are confident determine the refractive index of your unknown liquid. The
refractive index should be read to at least four decimal places.
- Since the laboratory
is not equipped with a constant temperature bath, the temp-erature of the
refractometer will probably be higher than 20°C. The nD values decrease as the temperature rises (nD is sometimes called “optical density”). To
compare your experimental value with the values given in Table #1, add
0.00045 for each degree above 20°C.
nD20 = nDT + ![]() (T – 20)(.00045).
(T – 20)(.00045).
Using the data collected for density, boiling point, and refractive index of the unknown liquid identify the unknown liquid. See Table 1 below. If more than one unknown or if none of the unknowns agree with your data then your measurements were not accurate enough and should be repeated.
Table 1. Physical Properties of Some Common Liquids
|
|
Boiling Point (0C) |
Density (200C) (g/ml) |
ND20 |
|
65.0 |
0.7914 |
1.3288 |
|
|
Ethanol |
78.5 |
0.7893 |
1.3611 |
|
1-propanol |
97.4 |
0.8035 |
1.3850 |
|
2-propanol |
82.4 |
0.7855 |
1.3776 |
|
Methyl acetate |
57.0 |
0.9330 |
1.3593 |
|
Ethyl acetate |
77.1 |
0.9003 |
1.3723 |
|
Acetone |
56.2 |
0.7899 |
1.3588 |
|
2-butanone |
79.6 |
0.8054 |
1.3788 |
|
Hexane |
69.0 |
0.6600 |
1.3750 |
|
water |
100.0 |
0.9972 |
1.3330 |