
In this experiment you will determine the density of an unknown liquid. You will measure the mass of a known volume delivered from a pipet and use the expression:
D = M/V
where D = density, M = mass and V = volume to calculate the density of the unknown liquid.
(Notice that you need to use the mass of just the unknown liquid and also the volume of the unknown liquid to obtain its density. What are your experimental readings? How are the mass and volume of the unknown determined from the experimental readings?)
1) Obtain a l0.00 mL pipet (preferably a class A
pipet), a pipet bulb, a stoppered l25 mL Erlenmeyer flask, and an unknown from
the stockroom. 
2) RECORD THE NUMBER OF YOUR UNKNOWN BEFORE YOU BEGIN WORK!! The unknowns are solutions of salt dissolved in water.
3) Rinse the flask AND your pipet three
times with two to three milliliter portions of your unknown solution
DISCARDING THE RINSINGS. Wipe the outside of the flask and the stopper
thoroughly with a towel. Handle the flask and stopper with tongs while
making your weighings. NOTE: The proper way to do this is 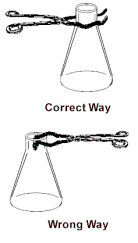 to put the tongs around the OUTSIDE of the
flask.
to put the tongs around the OUTSIDE of the
flask.
4) Weigh the stoppered flask on the Mettler Balance to 0.00l g. Record the mass on line 2 of the data sheet. Carefully pipet l0.00 mL of your unknown into the weighed flask. REREAD THE DIRECTIONS FOR THE USE OF THE PIPET BULB. Reweigh the flask (which now contains the unknown) and record the mass on line 1 of the data sheet.
5) Calculate the density of the solution.
6) Repeat the density determination as follows: Pour the liquid in your flask back into the sample bottle. DO NOT WASH THE FLASK (Why not?). Dry the outside of the flask thoroughly. Weigh the empty flask again (handling with tongs), record its mass, pipet l0.00 mL of the unknown into the flask and reweigh. Calculate the density from your second set of data. Make a third density determination in the same manner. Pour the solution from the third determination back into the sample bottle.
7) The results of the three determinations should agree within 0.l%. If one of your determinations is very different from the others you may disregard it when calculating your average density. If you know why the value is so different explain why on your report form. If all three of your determinations are similar use all three in calculating your average density.
8) At the end of the experiment wash the Erlenmeyer flask and the pipet with distilled water. Return the flask, the pipet and the pipet bulb to the stockroom. ALSO RETURN THE BOTTLE CONTAINING YOUR UNKNOWN TO THE STOCKROOM. DO NOT DISCARD THE LIQUID!!