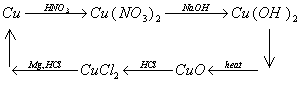
In this experiment, you are going to observe a sequence of reactions of copper that forms a cycle. You need to record observations and interpret them in the forms of chemical reaction equations. Also you need to classify the chemical reactions and study the quantitative relationships of the different reagent used in the reactions.
The cycle of copper reactions can be shown in an abbreviated diagram as below:
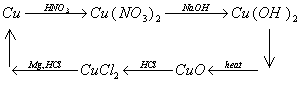
In detail, these reactions are
1) 4 HNO3(aq) + Cu(s) ==> Cu(NO3)2 (aq) + 2 NO2 (g) + 2 H2O (l)
2) Cu(NO3)2 (aq) + 2 NaOH (aq) ==> Cu(OH)2(s) + 2 NaNO3 (aq)
3) Cu(OH)2(s) + heat ==> CuO (s) + H2O (l)
4) CuO (s) + 2 HCl (aq) ==> CuCl2 (aq) + H2O (l)
5) CuCl2 (aq) + Mg(s) ==> MgCl2 (aq) + Cu(s)
These reactions provide you a chance to observe the different types of reactions including combination, decomposition, single displacement, double displacement and redox which are described in Silberberg’s chapter 4.
NaOH, HCl solutions are corrosive to the skin and especially dangerous if splashed into the eyes --- A reminder that you need to wear safety glasses, sneakers, and jeans.
Ethanol and acetone are flammable. You need to keep them away from all open flames.
2. Pipet 20 mL 0.100 M Cu(NO3)2 stock solution into a 100 mL beaker, add 6 mL of 2.0 M NaOH slowly while stirring gently with a glass stirring rod. The precipitate that forms is Cu(OH)2. What is formed in the solution besides Cu(OH)2? Record your observations.
3. Clamp an iron ring to the ring stand. Set the solution upon a wire-gauze supported by the iron ring. Heat it to nearly the boiling point while stirring it gently to prevent local over heating which may cause mixture to splatter. This will cause the decomposition of Cu(OH)2 to CuO. When the transformation is complete, remove the burner, continue stirring for two minutes, and then allow the CuO to settle. Record your observations.
4. Heat up 100 mL of distilled water in a 250-mL beaker to close to boiling using the same setup.
5. When the CuO precipitate settles well, decant (pour off) the supernatant liquid (the liquid portion above the settled CuO).
6. Add 50 mL of the now hot distilled water, allow to settle again, and then decant once more. Repeat this washing and decanting process with another 50 mL of the hot distilled water. What is removed by this washing and decanting process?
7. Add 4 mL of 6.0 M HCl while stirring. Record your observations. What is the solution now? Now transfer operations to the fume hood.
8. In the fume hood, add all at once .2 g of magnesium turning, stirring until the supernatant liquid is colorless. What happens? What is the gas produced? When the evolution of gas has become very slow, decant the supernatant liquid and pour it into the waste container provided.
9. If you can see any gray-silvery unreacted magnesium metal, add 2 mL of water and 2 mL of 6 M HCl. (You may warm (not boil) the solution on a hot plate in the fume hood to speed up the process.) When no hydrogen evolution can be detected, decant the supernatant liquid and transfer the copper to an weighed evaporating dish with a glass stirring rod.
10. Wash the product with 4 mL of distilled water, allow it to settle, and decant the wash water. Repeat the washing and decanting one more time. Then repeat the same washing procedures with first 4 mL ethanol and then 4 mL acetone. Place the evaporating dish under a heating lamp and dry the copper metal. What color is the metal? Record your observations and weigh the recovered copper.
11. Weigh the evaporating dish with the recovered copper. Calculate the theoretical, actual and percent yield of the copper product.