Observing
Living Daphnia
Living Daphnia were
observed with microscopes by placing the tiny crustacea in water filled
depression slides. Each depression slide acted like a tiny aquarium that
could be placed on the stage of a light microscope.
|
Experimental Set
Up:
-
We obtained a
culture of the microcrustacea Daphnia and observed it
under a dissecting microscope.
-
We obtained a
depression slide and coverslip. With a spoon we transferred
a waterflea (Daphnia) to the depression in the slide and filled
the depression with water from the culture. We placed the coverslip
over the depression capturing the waterflea so that it couldn't
swim around.
- We observed the
waterflea at 35X magnification with a dissecting microscope to
become familiar with its anatomy.
|
|
|
Observations:
-
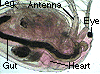
Observe the photograph
of the waterflea. Notice its antennae and legs. Their movement
establishes feeding currents and helps it respire.
-
Observe the photograph
of the waterflea. Note its heart which is located dorsally,
just behind its head and eye.Describe the egg cells.
|
|
Observing Daphnia
Heart Beats
A live Daphnia in
a depression slide was examined at high magnification to watch and count
its heart beats.
|
Experimental Set
Up:
-
We set up a compound
microscope and focused on the waterflea's heart at 160X magnification.
- We removed the
slide from the microscope being careful not to disturb the waterflea.
We set it on the countertop and allowed it to remain at room temperature
for 30 seconds.
|
 |
Observations:
-
Observe the photograph
of the waterflea's heart.
-
Note
the outline of the heart which has been enhanced so you can
get a good idea of how it looks.
|
 |
Observing Daphnia
in Freshwater
A live Daphnia in
a depression slide was examined in freshwater to determine its heart rate.
|
Experimental Set
Up:
-
We carefully
replaced the slide on the microscope, focused on the heart,
and videotaped the heart beating. We did not let the waterflea
remain on the microscope stage to long before starting the recording
to prevent the lamp from heating it up above room temperature.
-
We
determined the heart rate of the waterflea in freshwater by
counting the heart beats of the waterflea for 60 seconds and
graphed the heart volume versus time.
|
|
Observations:
-
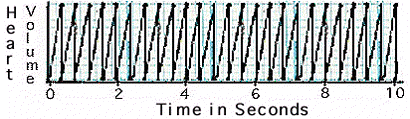
Observe the graph
of the waterflea's heart beat in freshwater.
-
Determine
the number of beats per minute.
|
|
|
Observing Daphnia
in Seawater
A live Daphnia in
a depression slide was covered with seawater and examined to determine
its heart rate.
|
Experimental Set
Up:
- We removed the
slide from the microscope and carefully removed the coverslip.
We removed the freshwater from the depression with an eyedropper
and refilled the depression with seawater (salinity=33 ppt). We
replaced the coverslip being careful not to squash the waterflea.
- We carefully replaced
the slide on the microscope, focused on the heart, and videotaped
the heart beating. We did not let the waterflea remain on the
microscope stage to long before starting the recording to prevent
the lamp from heating it up.
- We determined
the heart rate of the waterflea in seawater. Count the heart beats
of the waterflea for 60 seconds and graphed the heartbeats versus
time.
|
|
Observations:
-
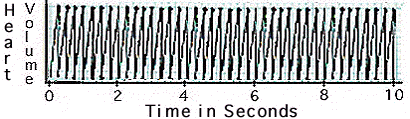
Observe the graph
of the waterflea's heart beat in seawater.
-
Determine
the number of beats per minute.
- How
does the waterflea's heart rate in seawater compare to its heart
rate in freshwater?
|
|
|
Observing Daphnia
in Hypersaline Water
A live Daphnia in
a depression slide was covered in hypersaline water and examined to determine
its heart rate.
|
Experimental Set
Up:
- We removed the
slide from the microscope and carefully removed the coverslip.
We removed the seawater from the depression with an eyedropper
and refilled the depression with hypersaline water (salinity=66
ppt). We replaced the coverslip being careful not to squash the
waterflea.
- We carefully replaced
the slide on the microscope, focused on the heart, and videotaped
the heart beating. We did not let the waterflea remain on the
microscope stage to long before starting the recording to prevent
the lamp from heating it up.
- We determined
the heart rate of the waterflea in hypersaline water. Count the
heart beats of the waterflea for 60 seconds and graphed the heart
beat versus time.
|
|
Observations:
-
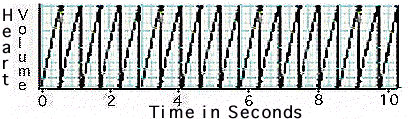
Observe the graph
of the waterflea's heart beat in hypersaline water.
-
Determine
the number of beats per minute.
- How
does the waterflea's heart rate in hypersaline water compare to
its heart rate in freshwater and in seawater?
|
|
|
|






